|
Katsumasa FUJITA
Ph. D.
Professor, Department of Applied Physics, University of Osaka
2-1 Yamadaoka, Suita, Osaka 565-0871, Japan
office: P2-300, Suita Campus, University of Osaka
phone: +81-6-6879-7847
email: fujita@ap.eng.osaka-u.ac.jp

|
|
BRI Workshop 2024
BRI Symposium 2019
Research Topics
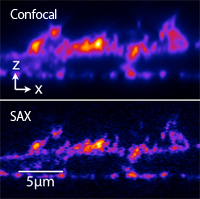
HeLa cell stained with AttoR6G phalloidin
|
Super-resolution fluorescence microscopy
We are developing optical microscopy techniques which can resolve fine structures of samples with the spatial resolution beyond the diffraction limit of light. By using saturated excitation or photoswiching of fluorescent molecules, we induce nonlinear fluorescence respoinse to realize super-resolution imaging. For example, saturated exction (SAX) miroscope can be realized by simply modulating laser intensity and demodulating fluorescence signal in a typical confocal microscope.
The figure shows the fluorescence images of a HeLa cell stained with AttoR6G phalloidin (xz cross-section). SAX microscopy can image fine structures that can not be resolved by conventional confocal microscopy.
References:
Temma et al., Nat. Methods (2024).
Temma et al., Opt. Express (2022).
Nawa et al., APL Photonics (2018).
Oketani et al., Opt. Lett. (2017)
Yonemaru et al., Phys. Rev. Applied (2015).
Chu et al., Phys. Rev. Lett. (2014).
Yamanaka et al., J. Biomed. Opt. (2013).
Fujita et al., Phys. Rev. Lett. (2007)
|
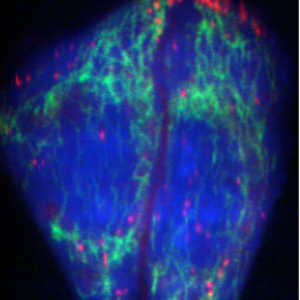
753 cm-1 : Cytochrome
1686 cm-1 : Protein
2852 cm-1 : Lipid
Movie by Raman
Cell division: cytokinesis
Cell division: mitosis
|
Raman microscopy for imaging of biological samples
We are developing Raman microscopes to image and analyze biomolecules in living cells. Raman scattering spectra show molecular vibrations in a sample, that contain rich information about species, conditions and environments of molecules. We have developed a slit-scanning Raman microscope and applied it to imaging of biomolecule dynamics in a living cell. We are also developing a higher-sensitive Raman detection technique for cellular imaging by using surface-enhanced Raman scattering and Raman tags to image small molecules.
The figure shows a Raman image of living HeLa cells. Molecular vibrations detected by Raman spectra produce the distribution of proteins and lipids in the cells.
References:
Bando et al., Biomed. Opt. Express. (2022).
SPIE Newroom (2016).
Wanatabe et al., Nat. Commun. (2015).
Palonpon et al, Nat. Protoc. (2013).
Okada et al., PNAS (2012).
Ando et al., Nano Lett. (2011).
Hamada et al., J. Biomed. Opt. (2008)
|
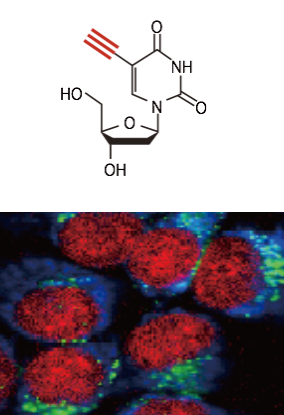
Alkyne (EdU), Cytochrome , Lipid
|
Raman tag imaging
Raman tag imaging is a new technique to observe small molecules in living cells and tissues. Small molecules are too "small" to be labeled by fluorescent dyes and could not be observed in the physiological conditions. We have demonstrated the use of Raman tag to visualize small molecules. Raman tag, such as an alkyne, shows a Raman band district from intracellular molecules and allows us to observe small molecules labeled by Raman tags with Raman microscopy. The Raman tag also realizes multiplexed imaging of many different molecules via the extremely narrow emission band of Raman scattering. We are also working on the application of the Raman tag technique for pharmaceutical researches.
References:
Dodo et al, JACS (2022).
Koike et al, ACS Nano (2020).
Ando et al, JACS (2016).
Ando et al, PNAS (2015).
Yamakoshi et al, Bioorg. Med. Chem. Lett. (2015).
Yamakoshi et al, Chem Commun. (2014).
Palonpon et al, Nat. Protoc. (2013).
Yamakoshi et al., JACS (2011)
|
Original Papers
- K. Tsuji†, M. Yamanaka†, Y. Kumamoto, S. Tamura, W. Miyamura, T. Kubo, K. Mizushima, K. Kono, H. Hirano, M. Shiozaki, X. Zhao, H. Xi, K. Sugiura, S. Fukushima, T. Kunimoto, Y. Tanabe, K. Nishida, K. Mochizuki, Y. Harada, N.I. Smith, R. Heintzmann, Z. Yu, M.C. Wang, T. Nagai, H. Tanaka, K. Fujita, “Time-deterministic cryo-optical microscopy,” Light Sci. Appl., 14: 275 (2025). †equal contribution
- A. Pelissier, K. Hashimoto, K. Mochizuki, J.N. Taylor, J.-E. Clément, Y. Kumamoto, K. Fujita, Y. Harada*, T. Komatsuzaki*, "Beyond the Nucleus: Cytoplasmic Dominance in Follicular Thyroid Carcinoma Detection Using Single-Cell Raman Imaging across Multiple Devices," Anal. Chem. 97 (25), 12998–13009 (2025).
- M. Lindley†, T. Kubo†, S. Devineau, M. Li, J. Qiao, T. Yashiro, S. Iwanaga, K. Moro, and K. Fujita, "Raman flow cytometry using time-delay integration," Optica, 12 (4) 479-489 (2025). †equal contribution
- K. Mizushima, Y. Kumamoto, S. Tamura, M. Yamanaka, K. Mochizuki, M. Li, S. Egoshi, K. Dodo, Y. Harada, N. I. Smith, M. Sodeoka, H. Tanaka, and K. Fujita, "Raman microscopy of cryofixed biological specimens for high-resolution and high-sensitivity chemical imaging," Sci. Adv., 10, eadn0110 (2024).
- M. Okada, K. Bando, Y. Shimaoka, Y. Nawa, K. Okada, S. Fujita, K. Fujita, and S. Iwanaga, "Rapid Surface-Enhanced Raman Scattering Imaging and Deep Learning for Highly Sensitive Discrimination of Amino Acids and Peptides," J. Phys. Chem. C, 128 (47) 20195–20204 (2024).
- J. N. Taylor, K. Bando, S. Tsukagoshi, L. Tanaka, K. Fujita, and S. Fujita, "Microscopic water dispersion and hydrogen-bonding structures in margarine spreads with Raman hyperspectral imaging and machine learning," Food Chem. 465 (2) 142035 (2025).
- J. Li, M. Li, Y. Nawa, Y. Liu, K. Bando, Y. Hua, L. Sun, S. Fujita, Y. Sawa, K. Fujita, and L. Liu, "Label-free Raman spectroscopy for assessing purity and maturationof hiPSC-derived cardiac tissue," Anal. Chem., 96 (39) 15765–15772 (2024).
- J. Okuda, N. Watanabe, T. Nakamura, K. Mizushima, H. Xi, Y. Kumamoto, K. Fujita, and M. Kino-Oka, "The impact of repeated temperature cycling on cryopreserved human iPSC viability stems from cytochrome redox state changes," Front. Bioeng. Biotechnol. 12:1443795 (2024).
- K. Temma†, R, Oketani†, T. Kubo, K. Bando, S. Maeda, K. Sugiura, T. Matsuda, R. Heintzmann, T. Kaminishi, K. Fukuda, M. Hamasaki, T. Nagai, and K. Fujita, "Selective-plane-activation structured illumination microscopy," Nat. Methods, 21, 889–896 (2024). †equal contribution
- A. J. Hobro, N. Pavillon, K. Koike, T. Sugiyama, T. Umakoshi, P. Verma, K. Fujita, and N. I. Smith, "Imaging vs Nonimaging Raman Spectroscopy for High-Throughput Single-Cell Phenotyping," Anal. Chem., 96, 18, 7047–7055 (2024).
- K. Tabata, H. Kawagoe, J. N. Taylor, K. Mochizuki, T. Kubo, J.-E. Clement, Y. Kumamoto, Y. Harada, A. Nakamura, K. Fujita, and T. Komatsuzaki, "On-the-fly Raman microscopy guaranteeing the accuracy of discrimination," Proc. Natl. Acad. Sci. USA, 121 (12) e2304866121 (2024).
- T. Hayashi, N. Ito, K. Tabata, A. Nakamura, K. Fujita, Y. Harada, T. Komatsuzaki, "Gaussian process classification bandits," Pattern Recognition, 149, 110224 (2024).
- H. Yamakoshi, D. Shibata, K. Bando, S. Kajimoto, A. Kohyama, S. Egoshi, K. Dodo, Y. Iwabuchi, M. Sodeoka, K. Fujita, T. Nakabayashi, "Ratiometric analysis of reversible thia-Michael reactions using nitrile-tagged molecules by Raman microscopy," Chem. Commun. 59, 14563-14566 (2023).
- H.-X. Liao, K. Bando, M. Li, K. Fujita, "Multifocal Raman spectrophotometer for examining drug-Induced and chemical-Induced cellular changes in 3D cell spheroids," Anal. Chem. 95 (39), 14616–14623 (2023).
- J. N. Taylor, A. Pélissier, K. Mochizuki, K. Hashimoto, Y. Kumamoto, Y. Harada, K. Fujita, T. Bocklitz, T. Komatsuzaki, "Correction for Extrinsic Background in Raman Hyperspectral Images," Anal. Chem., 95 (33) 12298-12305 (2023).
- K. Temma, M. Wincott, K. Fujita, and M. J. Booth, "Deflectometry based calibration of a deformable mirror for aberration correction and remote focusing in microscopy," Opt. Express., 31 (17) 28503-28514 (2023).
- A. H. Bhuiyan, J. Clement, Z. Ferdous, K. Mochizuki, K. Tabata, J. N. Taylor, Y. Kumamoto, Y. Harada, T. Bocklitz, K. Fujita, and T. Komatsuzaki, "Differentiability of cell types enhanced by detrending non-homogeneous pattern in line-illumination Raman microscope," Analyst, 148, 3574-3583 (2023).
- M. Li, Y. Ueyama-Toba, M. Lindley, G. Kongklad, Y. Nawa, Y. Kumamoto, S. Ishida, Y. Kanda, S. Fujita, H. Mizuguchi, K. Fujita, "Label-free evaluation of maturation and hepatotoxicity of human iPSC-derived hepatocytes using hyperspectral Raman imaging," Anal. Chem., 95 (24), 9252–9262 (2023).
- T. Li, J. Liu, M. Guo, F-C. Bin, J-Y. Wang, A. Nakayama, W-C. Zhang, F. Jin, X-Z. Dong, K. Fujita, M-L. Zheng, "Synthesis of biocompatible BSA-GMA and two-photon polymerization of 3D hydrogels with free radical type I photoinitiator," Int. J. Bioprinting. 9(5), 752 (2023).
- K. M. Helal, H. Cahyadi, J. N. Taylor, A. Okajima, K. Tabata, Y. Kumamoto, K. Mochizuki, Y. Itoh, T. Takamatsu, H. Tanaka, K. Fujita, T. Komatsuzaki, Y. Harada, "Raman imaging of rat non-alcoholic fatty liver tissues reveals distinct biomolecular states," FEBS Lett., 597, 1517-1527 (2023).
- K. Mochizuki, Y. Kumamoto, S. Maeda, M. Tanuma, A. Kasai, M. Takemura, Y. Harada, H. Hashimoto, H. Tanaka, N. I. Smith, and K. Fujita, "High-throughput line-illumination Raman microscopy with multislit detection," Biomed. Opt. Express., 14 (3), 1015-1026 (2023).
- M. Hayakawa, J. N. Taylor, R. Nakao, K. Mochizuki, Y. Sawai, K. Hashimoto, K. Tabata, Y. Kumamoto, K. Fujita, E. Konishi, S. Hirano, H. Tanaka, and T. Komatsuzaki, "Lipid Droplet Accumulation and Adipophilin Expression in Follicular Thyroid Carcinoma," Biochem. Biophys. Res. Commun., 640, 192-201 (2023).
- M. Li, Y. Nawa, S. Ishida, Y. Kanda, S. Fujita, and K. Fujita, "Label-free chemical imaging of cytochrome P450 activity by Raman microscopy," Commun Biol., 5, 778 (2022)
- M. Li, H.-X. Liao, K. Bando, Y. Nawa, S. Fujita, and K. Fujita, "Label-free monitoring of drug-induced cytotoxicity and its molecular fingerprint by live-cell Raman and autofluorescence imaging," Anal. Chem., 94 (28) 10019–10026 (2022).
- A. Nakayama, Y. Kumamoto M. Minoshima, K. Kikuchi, A. Taguchi, and K. Fujita, "Photoinitiator-free two-photon polymerization of biocompatible materials for 3D micro/nanofabrication,”Adv. Opt. Mater., 2200474 (2022).
- K. Bando, S. Yabuuchi, M. Li, T. Kubo, R. Oketani, N. I. Smith, and K. Fujita, "Bessel-beam illumination Raman microscopy,” Biomed. Opt. Express. 13 (6) 3161-3170 (2022).
- K. Temma, R. Oketani, R. Lachmann, T. Kubo, N. I. Smith, R. Heintzmann, and K. Fujita, "Saturated-excitation image scanning microscopy," Opt. Express. 30 (8) 13825-13838 (2022).
- K. Koike, N. I. Smith, and K. Fujita, “Spectral focusing in picosecond pulsed stimulated Raman scattering microscopy,” Biomed. Opt. Express., 13 (2), 995-1004 (2022).
- K. Nishida, H. Sato, R. Oketani, K. Mochizuki, K. Temma, Y. Kumamoto, H. Tanaka, K. Fujita*, "Using saturated absorption for super‐resolution laser scanning transmission microscopy," J. Microsc. 288 (2), 117-129 (2022).
- S. A. Hussain, T. Kubo, N. Hall, D. Gala, K. Hampson, R. Parton, M. A. Phillips, M. Wincott, K. Fujita, I. Davis, I. Dobbie, M. J. Booth, "Wavefront‐sensorless adaptive optics with a laser‐free spinning disk confocal microscope," J. Microsc., 288 (2), 106-116 (2022).
- H. Lee, H. Yoo, G. Moon, K.-A. Toh, K. Mochizuki, K. Fujita, and D. Kim, "Super-resolved Raman microscopy using random structured light illumination: concept and feasibility," J. Chem. Phys. 155, 144202 (2021).
- T. Kubo, K. Temma, K. Sugiura, H. Shinoda, K. Lu, N. I. Smith, T. Matsuda, T. Nagai, K. Fujita, "Visible-wavelength multiphoton activation confocal microscopy," ACS Photonics, 8 (9) 2666–2673 (2021).
- T. Ichimura, T. Kakizuka, K. Horikawa, K. Seiriki, A. Kasai, H. Hashimoto, K. Fujita, T. M. Watanabe, T. Nagai, “Exploring rare cellular activity in more than one million cells by a trans-scale scope,” Sci. Rep., 11, 16539 (2021).
- H. Kawagoe, J. Ando, M. Asanuma, K. Dodo, T. Miyano, H. Ueda, M. Sodeoka, K. Fujita, “Multiwell Raman plate reader for high-throughput biochemical screening,” Sci. Rep., 11, 15742 (2021).
- J. Ando, H. Kawagoe, A. Nakamura, R. Iino and K. Fujita, "Label-free monitoring of crystalline chitin hydrolysis by chitinase based on Raman spectroscopy," Analyst, 146, 4087-4094 (2021).
- F. Tai, K. Koike, H. Kawagoe, J. Ando, Y. Kumamoto, N. I. Smith, M. Sodeoka, K. Fujita, "Detecting nitrile-containing small molecules by infrared photothermal microscopy," Analyst, 146, 2307-2312 (2021).
- T. Kubo, K. Temma, N. I. Smith, K. Lu, T. Matsuda, T. Nagai, and K. Fujita, "Hyperspectral two-photon excitation microscopy using visible wavelength," Opt. Lett., 46 (1) 37–40 (2021).
- H. Lee, K. Kang, K. Mochizuki, C. Lee, K.-A. Toh, S. Lee, K. Fujita, D. Kim, "Surface plasmon localization-based super-resolved Raman microscopy," Nano Lett., 20 (12) 8951–8958 (2020).
- K. Koike, K. Bando, J. Ando, H. Yamakoshi, N. Terayama, K. Dodo, N. I. Smith, M. Sodeoka, K. Fujita, "Quantitative drug dynamics visualized by alkyne-tagged plasmonic-enhanced Raman microscopy," ACS NANO, 14(11), 15032–15041 (2020).
- A. Taguchi, A. Nakayama, R. Oketani, S. Kawata, K. Fujita, “Multiphoton-excited deep ultraviolet photolithography for 3D nanofabrication,” ACS Appl. Nano Mater., 3(11), 11434–11441 (2020).
- Y.-S. Duh, Y. Nagasaki, Y.-L. Tang, P.-H. Wu, H.-Y. Cheng, T.-H. Yen, H.-X. Ding, K. Nishida, I. Hotta, J.-H. Yang, Y.-P. Lo, K.-P. Chen, K. Fujita, C.-W. Chang, K.-H. Lin, J. Takahara, and S.-W. Chu, "Giant photothermal nonlinearity in a single silicon nanostructure." Nat Commun 11, 4101 (2020).
- K. Nishida, G. Deka, N. I. Smith, S.-W. Chu*, K. Fujita*, "Nonlinear scattering of near-infrared light for imaging plasmonic nanoparticles in deep tissue," ACS Photonics, 7, 2139–2146 (2020).
- K. Bando, Z. Zhang, D. Graham, K. Faulds, K. Fujita*, S. Kawata, "Dynamic pH measurement of intracellular pathways using nano-plasmonic assemblies," Analyst, 145, 5768 - 5775 (2020).
- M. Tanuma, A. Kasai, K. Bando, N. Kotoku, K. Harada, M. Minoshima, K. Higashino, A. Kimishima, M. Arai, Y. Ago, K. Seiriki, K. Kikuchi, S. Kawata, K. Fujita. and H. Hashimoto, “Direct visualization of an antidepressant analog using surface-enhanced Raman scattering in the brain,” JCI Insight, 5(6), e133348 (2020).
- R. Oketani, H. Suda, K. Uegaki, T. Kubo, T. Matsud, M. Yamanaka, Y. Arai, N. I. Smith, T. Nagai, K. Fujita, "Visible-wavelength two-photon excitation microscopy with multifocus scanning for volumetric live-cell imaging," J. Biomed. Opt., 25(1), 014502 (2020).
- Y. Nagasaki, T. Kohno, K. Bando, H. Takase, K. Fujita, J. Takahara, "Adaptive camouflage using VO2 optical antennas with subwavelength resolution," J. Phys. Chem. B, 123 (20) 4358-4372 (2019).
- Y. Kumamoto, K. Mochizuki, K. Hashimoto, Y.Harada, H. Tanaka, K. Fujita*, "High-Throughput cell imaging and classification by narrowband and low-spectral-resolution Raman microscopy," J. Phys. Chem. B., 123 (12), 2654 (2019).
- T. Morimoto, L.-d. Chiu*, H. Kanda, H. Kawagoe, T. Ozawa, M. Nakamura, K. Nishida, K. Fujita*, T. Fujikado, "Using redox-sensitive mitochondrial cytochrome Raman bands for label-free detection of mitochondrial dysfunction," Analyst, 144, 2540 (2019).
- Z. Zhang*, K. Bando, K. Mochizuki, A. Taguchi, K. Fujita*, and S. Kawata, "Quantitative evaluation of SERS nanoparticles for intracellular pH sensing at a single particle level," Anal. Chem., 91 (5), 3254 (2019).
- Y. Nawa, Y. Yonemaru, A. Kasai, R. Oketani, H. Hashimoto, N. I. Smith, and K. Fujita*, "Saturated excitation microscopy using differential excitation for efficient detection of nonlinear fluorescence signals," APL Photonics 3, 080805 (2018).
- A. Germond, T. Ichimura, L.-d. Chiu, K. Fujita, T. M. Watanabe, H. Fujita, "Cell type discrimination based on image features of molecular component distribution," Sci Rep., 8, 11726 (2018).
- G. Deka, K. Nishida, K. Mochizuki, H.-X. Ding, K. Fujita*, and S.-W. Chu*, “Resolution enhancement in deep-tissue nanoparticle imaging based on plasmonic saturated excitation microscopy,” APL Photonics 3, 031301 (2018).
- A. Doi, R. Oketani, Y. Nawa, and K. Fujita, “High-resolution imaging in two-photon excitation microscopy using in situ estimations of the point spread function”,Biomed. Opt. Express 9, 202 (2018).
- Z. Zhang*, K. Bando, A. Taguchi, K. Mochizuki, K. Sato, H. Yasuda, K. Fujita*, and S. Kawata, “Au-Protected Ag Core/Satellite Nanoassemblies for Excellent Extra-/Intracellular Surface-Enhanced Raman Scattering Activity”, ACS Appl. Mater. Interfaces, 9 (50), 44027 (2017).
- K. Seiriki, A. Kasai, T. Hashimoto, W. Schulze, M. Niu, S. Yamaguchi, T. Nakazawa, K. Inoue, S. Uezono, M. Takada, Y. Naka, H. Igarashi, M. Tanuma, J. A. Waschek, Y. Ago, K. F. Tanaka, A. Hayata-Takano, K. Nagayasu, N. Shintani, R. Hashimoto, Y. Kunii, M. Hino, J. Matsumoto, H. Yabe, T. Nagai, K. Fujita, T. Matsuda, K. Takuma, A. Baba, H. Hashimoto, "High-Speed and Scalable Whole-Brain Imaging in Rodents and Primates," Neuron 94, 1085 (2017).
- L.-d. Chiu, T. Ichimura, T. Sekiya, H. Machiyama, T. Watanabe, H. Fujita, T. Ozawa, K. Fujita*, "Protein expression guided chemical profiling of living cells by hybrid fluorescence-Raman microscopy," Sci. Rep. 7: 43569 (2017).
- R. Oketani, A. Doi, N. I. Smith, Y. Nawa, S. Kawata, and K. Fujita*, "Saturated two-photon excitation fluorescence microscopy with core-ring illumination," Opt. Lett., 42 (3) 571 (2017).
- T. Ichimura , L.-d. Chiu , K. Fujita , H. Machiyama , T. Yamaguchi , T. Watanabe, H. Fujita, "Non-label immune cell state prediction using Raman spectroscopy," Sci. Rep. 6: 37562 (2016).
- J. Ando, M. Asanuma, K. Dodo, H. Yamakoshi, S. Kawata, K. Fujita* and M. Sodeoka*, "Alkyne-tag SERS screening and identification of small-molecule-binding sites in protein," J. Am. Chem. Soc., 38 (42) 13901 (2016).
- Y.-T. Chen, P.-H. Lee, P.-T. Shen, J. Launer, R. Oketani, K.-Y. Li, Y.-T. Huang, K. Masui, S. Shoji, K. Fujita, S.-W. Chu, “Study of nonlinear plasmonic scattering in metallic nanoparticles,” ACS Photonics, 3 (8), 1432–1439 (2016).
- H.-Y. Wu, Y.-T. Huang, P.-T. Shen, H. Lee, R. Oketani, Y. Yonemaru, M. Yamanaka, S. Shoji, S. Kawata, C.-W. Chang, K.-H. Lin, K. Fujita, S.-W. Chu, "Ultrasmall all-optical plasmonic switch and its application to superresolution imaging," Sci. Rep., 6: 24293 (2016).
- H. Lee, K.-Y. Li, Y.-T. Huang, P.-T. Shen, G. Deka, R. Oketani, Y. Yonemaru, M. Yamanaka, K. Fujita, S.-W. Chu, "Measurement of scattering nonlinearities from a single plasmonic nanoparticle," J. Vis. Exp. (107), e53338 (2016).
- Y. Kumamoto, K. Fujita, N. I. Smith, and S. Kawata, "Deep-UV biological imaging by lanthanide ion molecular protection," Biomed. Opt. Express, 7 (1), 158-170 (2016).
- K. Watanabe, A. F. Palonpon, N. I. Smith, L.-d. Chiu, A. Kasai, H. Hashimoto, S. Kawata, K. Fujita, "Structured line illumination Raman microscopy," Nat. Commun. 6:10095 (2015).
- K. Bando, N. I. Smith, K. Fujita, S. Kawata, "Analysis of dynamic SERS spectra measured with a nanoparticle during intracellular transportation in 3D," J. Opt., 17, 114023 (2015).
- M. Yamanaka, K. Saito, N. I. Smith, Y. Arai, K. Uegaki, Y. Yonemaru, K. Mochizuki, S. Kawata, T. Nagai, K. Fujita, "Visible-wavelength two-photon excitation microscopy for fluorescent protein imaging," J. Biomed. Opt., 20 (10), 101202 (2015).
- A. Hashimoto, Y. Yamaguchi, L.-d. Chiu, C. Morimoto, K. Fujita, M. Takedachi, S. Kawata, S. Murakami, E. Tamiya, "Time-lapse Raman imaging of osteoblast differentiation," Sci. Rep., 5, 12529 (2015).
- Y. Yonemaru, A. F. Palonpon, S. Kawano, N. I. Smith, S. Kawata, and K. Fujita, "Super-spatial- and -spectral-resolution in vibrational imaging via saturated coherent anti-Stokes Raman scattering," Phys. Rev. Applied, 4, 014010 (2015).
- Z. Zheng, S. Mizukami, K. Fujita, and K. Kikuchi, "An enzyme-responsive metal-enhanced near-infrared fluorescence sensor based on functionalized gold nanoparticles," Chem. Sci., 6, 4934-4939 (2015).
- T. Ichimura, L.-d. Chiu, K. Fujita, H. Machiyama, S. Kawata, T. M. Watanabe, H. Fujita, "Visualizing the appearance and disappearance of the attractor of differentiation using Raman spectral imaging," Sci. Rep., 5, 11358 (2015).
- D. K. Tiwari, Y. Arai, M. Yamanaka, T. Matsuda, M. Agetsuma, M. Nakano, K. Fujita, T. Nagai, "Fast positively photoswitchable fluorescent protein for ultra-low laser power RESOLFT nanoscopy," Nat. Methods, 12, 515-518 (2015).
- J. Ando, M. Kinoshita, J. Cui, H. Yamakoshi, K. Dodo, K. Fujita, M. Murata, M. Sodeoka, "Sphingomyelin distribution in lipid rafts of artificial monolayer membranes visualized by Raman microscopy," Proc. Natl. Acad. Sci., 112 (15), 4558-4563 (2015).
- K. Mochizuki, L. Shi, S. Mizukami, M. Yamanaka, M. Tanabe, W.-T. Gong, A. F. Palonpon, S. Kawano, S. Kawata, K. Kikuchi, K. Fujita, "Nonlinear fluorescence imaging by photoinduced charge separation," Jpn. J. Appl. Phys., 54, 042403 (2015).
- A. J. Hobro, N. Pavillon, K. Fujita, M. Ozkan, C. Coban, N. I. Smith, "Label-free Raman imaging of the macrophage response to the malaria pigment hemozoin," Analyst, 140, 2350-2359 (2015).
- Y. Saito and K. Fujita, "Direct electron density modulation of surface plasmons with a scanning electron microscope," Appl. Phys. Express 8, 015001 (2015).
- H. Yamakoshi, A. Palonpona, K. Dodo, J. Ando, S. Kawata, K. Fujita, M. Sodeoka, "A sensitive and specific Raman probe based on bisarylbutadiyne for live cell imaging of mitochondria," Bioorg. Med. Chem. Lett. 25 (3), 664-667 (2015).
- L.-d. Chiu, A. F. Palonpon, N. I. Smith, S. Kawata, M. Sodeoka, K. Fujita, "Dual-polarization Raman spectral imaging to extract overlapping moleclular fingerprints of living cells," J. Biophotonics, 8 (7), 546-6554 (2015).
- H. Lee, R. Oketani, Y.-T. Huang, K.-Y. Li, Y. Yonemaru, M. Yamanaka, S. Kawata, K. Fujita, S.-W. Chu, "Point spread function analysis with saturable and reverse saturable scattering," Opt. Express, 22 (21), 26016 (2014).
- N. I. Smith, K. Mochizuki, H. Niioka, S. Ichikawa, N. Pavillon, A. J. Hobro, J. Ando, K. Fujita, Y. Kumagai, "Laser-targeted photofabrication of gold nanoparticles inside cells," Nat. Commun., 5, 5144 (2014).
- K.-C. Huang, K. Bando, J. Ando, N. I. Smith, K. Fujita, S. Kawata, "3D SERS (surface enhanced Raman scattering) imaging of intracellular pathways," Methods, 68 (2), 348 (2014).
- Y. Yonemaru, M. Yamanaka, N. I. Smith, S. Kawata, K. Fujita, "Saturated excitation microscopy with optimized excitation modulation," ChemPhysChem, 15 (4), 743 (2014).
- A. Hashimoto, L.-d. Chiu, K. Sawada, T. Ikeuchi, K. Fujita, M. Takedachi, Y. Yamaguchi, S. Kawata, S. Murakami, E. Tamiya, "In situ Raman imaging of osteoblastic mineralization," J. Raman Spectrosc., 45 (2), 157 (2014).
- S.-W. Chu, T.-Y. Su, R. Oketani, Y.-T. Huang, H.-Y. Wu, Y. Yonemaru, M. Yamanaka, H. Lee, G.-Y. Zhuo, M.-Y. Lee, S. Kawata, and K. Fujita, "Measurement of a saturated emission of optical radiation from gold nanoparticles: application to an ultrahigh resolution microscope," Phys. Rev. Lett., 112 (1), 017402 (2014). (highlighted in APS physics spotlights)
- T. Ichimura, L.-d. Chiu, K. Fujita, S. Kawata, T. M. Watanabe, T. Yanagida, H. Fujita, "Visualizing cell state transition using Raman spectroscopy," PLOS ONE, 9 (1), e84478 (2014).
- S.-W. Chu, H.-Y. Wu, Y.-T. Huang, T.-Y. Su, H. Lee, Y. Yonemaru, M. Yamanaka, R. Oketani, S. Kawata. S. Shoji, and K. Fujita, "Saturation and reverse saturation of scattering in a single plasmonic nanoparticle," ACS Photonics, 1 (1), 32-37 (2014). (introduced in Nature Photonics, 8, 92 (2014).
- H. Yamakoshi, A. F. Palonpon, K. Dodo, J. Ando, S. Kawata, K. Fujita, M. Sodeoka, "Simultaneous imaging of protonated and deprotonated carbonylcyanide p-trifluoromethoxyphenylhydrazone in live cells by Raman microscopy," Chem Commun., 50, 1341 (2014).
- M. Yamanaka, Y. Yonemaru, S. Kawano, K. Uegaki, N. I. Smith, S. Kawata, K. Fujita, "Saturated excitation microscopy for sub-diffraction-limited imaging of cell clusters," J. Biomed. Opt., 18 (12), 126002 (2013).
- M. Yamanaka, K. Saito. N. I. Smith, S. Kawata, T. Nagai, K. Fujita, "Saturated excitation (SAX) of fluorescent proteins for sub-diffraction-limited imaging of living cells in three dimensions," Interface FOCUS, 3, 20130007 (2013).
- A. F. Palonpon, J. Ando, H. Yamakoshi, K. Dodo, M. Sodeoka, S. Kawata, K. Fujita, "Raman and SERS microscopy for molecular imaging of live cells," Nat. Protoc., 8, 677 (2013).
- T. Shimozawa, K. Yamagata, T. Kondo, S. Hayashi, A. Shitamukai, D. Konno, F. Matsuzaki, J. Takayama, S. Onami, H. Nakayama, Y. Kosugi, T. M. Watanabeg, K. Fujita, Y. Mimori-Kiyosue, "Improving spinning disc confocal microscopy by preventing pinhole cross-talk for intravital imaging," Proc. Nat. Acad. Sci. USA., 110 (9), 3399 (2013).
- N. Pavillon, K. Bando, K. Fujita, and N. I. Smith, "Feature-based recognition of Surface-enhanced Raman spectra for biological targets," J. Biophoton., 6 (8), 587 (2013).
- M. Okada, N. I. Smith, A. F. Palonpon, H. Endo, S. Kawata, M. Sodeoka, K. Fujita, "Label-free Raman observation of cytochrome c dynamics during apoptosis," Proc. Natl. Acad. Sci. USA, 109 (1) 28-32 (2012).
- H. Yamakoshi ,K. Dodo , A. F. Palonpon , J. Ando , K. Fujita , S. Kawata, and M. Sodeoka, "Alkyne-tag Raman imaging for visualization of mobile small molecules in live cells," J. Am. Chem. Soc., 134, 20681-20689 (2012).
- Y. Masago, A. Hosoya, K. Kawasaki, S. Kawano, A. Nasu, J. Toguchida, K. Fujita, H. Nakamura, G. Kondoh, and K. Nagata, "Molecular chaperone Hsp47 is essential for cartilage and endochondral bone formation," J. Cell Science, 125 (Pt 5), 1118-1128 (2012).
- J. Ando, K. Fujita, N. I. Smith and S. Kawata, "Dynamic SERS imaging of cellular transport pathways with endocytosed gold nanoparticles," Nano Lett., 11 (12), 5344-5348 (2011).
- M. Yamanaka, Y-. K. Tzeng, S. Kawano, N. I. Smith, S. Kawata, H-. C. Chang, and K. Fujita, "SAX microscopy with fluorescent nanodiamond probes for high-resolution fluorescence imaging," Biomed. Opt. Express, 2 (7), 1946-1954 (2011)
- M. Honda, Y. Saito, N. I. Smith, K. Fujita, and S. Kawata, "Nanoscale heating of laser irradiated single gold nanoparticles in liquid," Opt. Express, 19 (13), 12375-12383 (2011).
- M.-L. Zheng, K. Fujita, W.-Q. Chen, X.-M. Duan, and S. Kawata, "Two-photon excited fluorescence and second-harmonic generation of the DAST organic nanocrystals," J. Phys. Chem. C, 115 (18), 8988-8993 (2011).
- H. Yamakoshi, K. Dodo, M. Okada, J. Ando, A. Palonpon, K. Fujita, S. Kawata, and M. Sodeoka, "Imaging of EdU, an alkyne-tagged cell proliferation probe, by Raman microscopy," J. Am. Chem. Soc., 133 (16), 6102–6105 (2011).
- S. Kawano, N. I. Smith, M. Yamanaka, S. Kawata and K. Fujita, "Determination of the expanded optical transfer function in saturated excitation imaging and high harmonic demodulation," Appl. Phys. Express, 4, 042401 (2011).
- M.-L. Zheng, K. Fujita, W.-Q. Chen, N. I. Smith, X.-M. Duan, and S. Kawata, "Comparison of staining selectivity of subcellular structures by carbazole-based cyanine probes for nonlinear optical microscopy," ChemBioChem., 12, 52-55 (2011).
- R. J. Milewski, Y. Kumagai, K. Fujita, D. M. Standley, N. I. Smith, "Automated processing of label-free Raman microscope images of macrophage cells with standardized regression for high-throughput analysis," Immunome Research, 6, 11 (2010).
- M.-L. Zheng, W.-Q. Chen, K. Fujita, X.-M. Duan, and S. Kawata, "Dendrimer adjusted nanocrystals of DAST: organic crystal with enhanced nonlinear optical properties," Nanoscale, 2, 913 - 916 (2010).
- T. Minamikawa, M. Hashimoto, K. Fujita, S. Kawata, and T. Araki, "Multi-focus excitation coherent anti-Stokes Raman scattering (CARS) microscopy and its applications for real-time imaging," Opt. Express, 17, 9526-9536 (2009). (selected for the publication in Virtual Journal for Biomedical Optics, 4 (8), 2009)
- K. Fujita, S. Ishitobi, K. Hamada, N. I. Smith, A. Taguchi, Y. Inouye, and S. Kawata, "Time-resolved observation of surface-enhanced Raman scattering from gold nanoparticles during transport through a living cell," J. Biomed. Opt. 14, 024038 (2009).
- M. Ogawa, Y. Harada, Y. Yamaoka, K. Fujita, H. Yaku, T. Takamatsu, "Label-free biochemical imaging of heart tissue with high-speed spontaneous Raman microscopy," Biochem. Biophys. Res. Communn., 382, 370-374 (2009).
- J. Ando, N. I. Smith, K. Fujita, and S. Kawata, "Photogeneration of membrane potential hyperpolarization and depolarization in non-excitable cells," Eur. Biophys. J., 38, 255-262 (2009).
- H. Ishitobi, T. Kai, K. Fujita, Z. Sekkat, and S. Kawata, "On fluorescence blinking of single molecules in polymers," Chem. Phys. Lett. 468, 234-238 (2009).
- J. Ando, G. Bautista, N. I. Smith, K. Fujita, and V. Daria, "Optical trapping and surgery of living yeast cells using a single laser," Rev. Sci. Instr., 79, 103705 (2008).
- M. Yamanaka, S. Kawano, K. Fujita, N. I. Smith, and S. Kawata, "Beyond the diffraction limit biological imaging by saturated excitation (SAX) microscopy," J. Biomed. Opt. 13, 050507 (2008).
- H. Niioka, N. I. Smith, K. Fujita, and S. Kawata, "Femtosecond laser nano-ablation in fixed and non-fixed cultured cells," Opt. Express, 16, 14476-14495 (2008).
- K. Hamada , K. Fujita, N. I. Smith , M. Kobayashi , Y. Inouye , S. Kawata, "Raman microscopy for dynamic molecular imaging of living cells," J. Biomed. Opt., 13, 044027 (2008).
- N. I. Smith, Y. Kumamoto, S. Iwanaga, J. Ando, K. Fujita, and S. Kawata, "A femtosecond laser pacemaker for heart muscle cells," Opt. Express, 16 (12) 8604-8616 (2008).
- Y. Saito, M. Kobayashi, D. Hiraga, K. Fujita, S. Kawano, N. I. Smith, Y. Inouye, S. Kawata, "Z-polarization sensitive detection in micro Raman spectroscopy by radially polarized incident light," J. Raman. Spectrosc., 39, 1643-1648 (2008).
- K. Fujita, M. Kobayashi, S. Kawano, M. Yamanaka, and S. Kawata, "High-resolution confocal microscopy by saturated excitation of fluorescence," Phys. Rev. Lett., 99, 228105 (2007). (selected for Virtual Journal of Biological Physics Research, 14 (11) 2007)
- T. Minamikawa, N. Tanimoto, M. Hashimoto, T. Araki, M. Kobayashi, K. Fujita and S. Kawata, "Jitter reduction of two synchronized picosecond mode-locked lasers using balanced cross-correlator with two-photon detectors," Appl. Phys. Lett. 89, 191101 (2006).
- S. Iwanaga, T. Kaneko, K. Fujita, N. Smith, O. Nakamura, T. Takamatsu, and S. Kawata, "Location-dependent Photogeneration of calcium waves in HeLa cells, "Cell Biochem. Biophys., 45 (2), 167-176 (2006).
- A. Fujita, K. Fujita, O. Nakamura, T. Matsuda, and S. Kawata, "Control of cardiomyocyte orientation on a microscaffold fabricated by photopolymerization with laser beam interference," J. Biomed. Opt., 11 (2), 021015 (2006).
- S. Iwanaga, N. I. Smith, K. Fujita, and S. Kawata, "Slow Ca2+ wave stimulation using low repetition rate femtosecond pulsed irradiation," Opt. Express, Vol. 14, pp.717-725 (2006).
- N. I. Smith, S. Iwanaga, T. Beppu, K. Fujita, O. Nakamura, and S. Kawata, "Photostimulation of two types of Ca2+ waves in rat pheochromocytoma PC12 cells by ultrashort pulsed near-infrared laser irradiation," Laser Physics Letters, 3 (3) 154-161 (2006).
- S. Iwanaga, N. Smith, K. Fujita, S. Kawata, and O. Nakamura, "Single pulse cell stimulation with a near-infrared picosecond laser", Appl. Phys. Lett., 87, 243901 (2005).
- T. Tanabe, M. Oyamada, K. Fujita, P. Dai, H. Tanaka, T. Takamatsu, "Multiphoton excitation-evoked chromophore-assisted laser inactivation using green fluorescent protein," Nat. Methods, 2,503-505 (2005).
- M. Kobayashi, K. Fujita, O. Nakamura, S. Kawata,"Time-gated imaging for multifocus second-harmonic generation microscopy,"Rev. Sci. Instru., 76, 073704 (2005). (elected for Virtual Journal of Ultrafast Science, 4 (8) 2005)
Review Articles and Others
- K. Fujita, "Raman imaging as a window into cellular complexity: a future perspective," Nature Methods, 22, 890–892 (2025).
- K. Dodo, W. J. Tipping, H. Yamakoshi, S. Egoshi, T. Kubo, Y. Kumamoto, K. Faulds, D. Graham, K. Fujita, and M. Sodeoka, "Alkyne-tag Raman imaging and sensing of bioactive compounds," Nat. Rev. Methods Primers, 5, Article number: 20 (2025).
- M. Li and K. Fujita, "Analytical Biology: An Emerging Discipline for the Future," Spectroscopy, 39 (2) pp. 28-31 (2024).
- V. Astratov, ... K. Fujita, et al., "Roadmap on Label-Free Super-Resolution Imaging"Laser Photonics Rev., 2200029 (2023).
- Y. Kumamoto, M. Li, K. Koike, and K. Fujita, "Slit-scanning Raman microscopy: Instrumentation and applications for molecular imaging of cell and tissue," J. Appl. Phys. 132, 171101 (2022).
- K. Dodo, K. Fujita, M. Sodeoka, "Raman spectroscopy for chemical biology research," J. Am. Chem. Soc. 144 (43) 19651–19667 (2022).
- Y.-L. Tang, T.-H. Yen, K. Nishida, J. Takahara, T. Zhang, X. Li, K. Fujita, and S.-W. Chu, "Mie-enhanced photothermal/thermo-optical nonlinearity and applications on all-optical switch and super-resolution imaging," Opt. Mater. Express 11(11), 3608-3626 (2021).
- J. Ando, K. Bando, K. Koike, K. Fujita, "Applications of Metallic Nanoparticles in Bio-imaging and Molecular Spectroscopy," Material Matters, 16(2) 18-22 (2021).
- G. Deka, C.-K. Sun, K. Fujita, S.-W. Chu, "Nonlinear plasmonic imaging techniques and their biological applications," Nanophotonics, Vol.6, Issue1, pp.31-49 (2017).
- K. Fujita, "Fast Raman imaging of living cells for biomedical applications," SPIE Newroom, 7 September (2016).
- K. Fujita, "Follow-up review: Recent progress in the development of super-resolution optical microscop," Microscopy, 65 (4) 275-281(2016).
- J. Ando, A. F. Palonpon, M. Sodeoka, K. Fujita, "High-speed Raman imaging of cellular processes," Curr. Opin. Chem. Biol., Vol.33, pp.16–24 (2016).
- M. Yamanaka, N. I. Smith, K. Fujita, "Introduction to super resolution microscopy," Microscopy, 63 (3) 177-192 (2014).
- N. Pavillon, K. Fujita, N. I. Smith, "Multimodal label-free microscopy," J. Innov. Opt. Health. Sci. 7 (5), 1330009-1~22 (2014)
- J. Ando, T. Yano, K. Fujita, S. Kawata, "Metallic nanoparticles for nanoimaging and nanoanalysis," Phys. Chem. Chem. Phys., 15, 13713-13722 (2013).
- A. F. Palonpon, M. Sodeoka, K. Fujita, "Molecular imaging of live cells by Raman microscopy," Curr. Opin. Chem. Biol., 17, 708-715 (2013).
- J. Ando and K. Fujita, "Metallic nanoparticles as SERS agents for biomolecular imaging," Curr Pharm Biotechnol. 14, 141-149 (2013).
- K. Fujita and N. I. Smith, "Label-free molecular imaging of living cells," Mol. Cells, 26, 530-535 (2008).
Invited Talks (International)
- K. Fujita, "Advancing Raman Microscopy for High-Speed, High-Sensitivity Biological Imaging," Focus on Microscopy (Taipei, 14 Apr 2025.
- K. Fujita, "Raman microscopy of rapidly frozen biological samples," Photonics West 2025 (San Francisco, 25 Jan 2025).
- K. Fujita, "Practical application of quick-freezing devices for optical observation," Osaka University DeepTech Showcase (Palo Alto, 4 Dec 2024).
- K. Fujita, "Low-temperature Raman imaging of cryofixed biological samples," International Society on Optics Within Life Sciences (OWLS-17) (Bombay, 18 Nov 2024).
- K. Fujita, "Practical implementation and industrial application of cryogenic optical microscopy," LAB TO MARKET: EXPLORING KANSAI'S TOP RESEARCH WITH KSAC (Singapore, 28 Oct 2024).
- K. Fujita, "Exploring extremes: fast and slow Raman microscopy for maximizing full spectroscopic potential," SCIX2024 (24 Oct 2024).
- K. Fujita, "ML-enhanced Raman microscopy for rapid and high-sensitivity molecular diagnosis," SCIX2024 (22 Oct 2024).
- K. Fujita, "High-sensitivity Raman imaging of cryofixed biological samples," ACS Fall Meeting (Denver, Aug 2024).
- K. Fujita, "Cryo-Raman Imaging: capturing the moment of biological activities," The XXVIII International conference on Raman spectroscopy (ICORS 2024) (Rome, 2 Aug 2024).
- K. Fujita, "Raman imaging: Principle, Application, and Issues," CHARISMA Raman School 2024 (Rome, 28 Jul 2024).
- K. Fujita, "Cryo-optical microscopy for multimodal molecular imaging," The Oxford International Conference on Advanced Optics and Photonics (Oxford, Julu 2024)
- K. Fujita, Cryo-optical microscopy for super-resolution imaging of the moment"," The 21st Congress of the International Union for Pure and Applied Biophysics (IUPAB2024) (Kyoto, Jun 2024).
- K. Fujita, "Raman microscopy: a new imaging modality for biology and medicine," OPTICA Student Chapter Lecture (Manipal, April 2024).
- K. Fujita, "Multimodal cryo-optical microscopy for high-sensitivity molecular imaging," Taiwan Japan Bilateral Symposium on Photonics 2024 (Taipei, 21 Feb 2024)
- K. Fujita, Kenta Mizushima, Shoko Tamura, Masahito Yamanaka, Menglu Li, Yoshinori Harada, Nicholas I. Smith, Yasuaki Kumamoto, Hideo Tanaka "Raman imaging of cryofixed cells with preservation of molecular states," SPIE Photonics West (San Francisco, 27 Jan 2024).
- K. Fujita, "Visualization of drug uptake and its cellular response by Raman microscopy," SPIE Photonics West (San Francisco, 27 Jan 2024).
- K. Fujita, "Unraveling of Raman Spectroscopy for Chemical Biology Research," ACS Monash University Malaysia International Student Chapter Seminar Series (Online, 22 Jan 2024).
- K. Fujita, "High-sensitiivity Raman imaging of cryofixed biological samples," RamanFest2023 (Paris, 9-10 November 2023).
- K. Fujita, "Enhancement of intraspheroid imaging through Bessel-beam side-illumination in Raman and structured illumination microscopy," International Forum on Microscopy 2023 (Zhongshan, 10 Septmber 2023).
- K. Fujita, "High-throughput Raman microscopy for detecting intracellular molecules," The 12th Asia-Pacific Laser Symposium (Hakodate, 5 September 2023).
- K. Fujita, "Hyperspectral Raman microscopy: toward visualization of intracellular chemistry," Gordon Research Conference, Chemical Imaging (Easton, 31 July 2023).
- K. Fujita, "Improvement of the detection sensitivity in Raman microscopy," Biomedical Raman Imaging 2023 (Atlanta, 27 June 2023).
- K. Fujita, "Raman microscopy: a new imaging modality for biology and medicine," OIST-OU Joint Symposium, A recipe for scientific synergy -Series 4- “Advancing biotechnology through multidisciplinary approaches” (Suita, 29 May 2023).
- K. Fujita, "Side-illumination Raman microscopy using a Bessel beam for observation of cell spheroids," The 5th Global Conference on Biomedical Engineering (Taipei, 16 Dec 2023).
..... and more
Books
- H. Yamakoshi, J. Ando, S. Egoshi, K. Dodo, M. Sodeoka, K. Fujita, "Raman Imaging and Screening of Bioactive Small Molecules," Raman Spectroscopy in Human Health and Biomedicine (H. Sato et al Ed, WSPC, September 2023)
- H. Yamakoshi, K. Fujita, “Spontaneous Raman and SERS microscopy for Raman tag imaging,” in Stimulate Raman Scattering Microscopy, pp. 275-287 (J.-X. Chen et al Ed, Elsevier 2021).
- K. Fujita, "Super-Resolution Imaging in Raman Microscopy" in Label-Free Super-Resolution Microscopy, Astratov Ed. (Springer Nature, 2019).
- K. Mochizuki, N. I. Smith, K. Fujita, "Raman microscopy," Reference Module in Chemistry, Molecular Sciences and Chemical Engineering (Elsevier)
- J. Ando, K. Dodo, K. Fujita, M. Sodeoka, "Visualizing Bioactive Small Molecules by Alkyne Tagging and Slit-Scanning Raman Microscopy, "Visualizing bioactive small molecules by alkyne tagging and slit-scanning Raman microscopy," Systems Chemical Biology: Methods and Protocols, Methods in Molecular Biology, Vol.1888, pp. 99-114 (Springer Nature 2019).
- K. Fujita, "Micro-Raman Spectroscopy," in Compendium of Surface and Interface Analysis, pp. 375-379 (Springer Singapore, 2018).
- K. Fujita, K. Mochizuki, N. I. Smith, "SAX microscopy and its application to imaging of 3D cultured cells," in Super-resolution imaging in biomedicine (A. Diaspro and M. A. M. J. van Zandvoort Eds., CRC Press, October 14, 2016).
- M. Hashimoto, T. Ichimura, K. Fujita, "CARS Microscopy: Implementation of Nonlinear Vibrational Spectroscopy for Far-Field and Near-Field Imaging," in Raman Imaging, Springer Series in Optical Sciences 168 (A. Zoubir Ed., Springer-Verlag Berlin Heidelberg 2012).
- Yasuaki Kumamoto, Nicholas Isaac Smith, Katsumasa Fujita, Jun Ando and Satoshi Kawata, "Optical Techniques for Future Pacemaker Technology," in Modern Pacemakers - Present and Future (M. K. Da ed. InTech 2011).
- N. I. Smith, S. Kawano, M. Yamanaka, and K. Fujita, "Nonlinear Fluorescence Imaging by Saturated Excitation," in
Nanoscopy and Multidimensional Optical Fluorescence Microscopy, pp. 2-1 ~ 2-16
(A. Diaspro Ed., Chapman and Hall/CRC press, April 2010).
- P. Verma, K. Fujita, T. Ichimura, and S. Kawata, "Raman, CARS and near-field Raman-CARS microscopy for cellular and molecular imaging," in "Nano Biophotonics - Science and Technology -," pp. 57-71
(H. Masuhara, S. Kawata, and F. Tokunaga Ed. Elservier B.V., Amsterdam, 2007).
- K. Fujita, N. Smith, and O. Nakamura,
"Nonlinear optical imaging and stimulation of living cells," in
Nanophotonics -Intengrating photochemistry, Optics and Nano/Bio Materials Studies -
(H. Masuhara and S. Kawata Ed. Elsevier B.V., Amsterdam, 2004).
- K. Fujita and T. Takamatsu,
"Real-time in situ calcium imaging with single and two-photon confocal microscopy," in
Confocal and two-photon microscopy: Foundation, Application and Advances
(A. Diaspro Ed. John Wiley & Sons, Inc., New York, 2001).
..... and more
Award
Miscellaneous
- "Technology development could bring Raman microscopy to the clinic," Optica New Release, Feburuary 6, 2023
- “A further leap of biomedical Raman imaging,” Spectroscopy, Vol. 35, Issue 7, p.10 (2020).
- “Technology Feature, Live-cell imaging: Deeper, faster, wider,” Science, Vol. 359, Issue 6383, pp. 1549 (2018).
- "Raman Imaging of Live Cells," Spectroscopy, 18 Nov (2014).
- "Harmonic Microscopy," RIKEN Research 14 Mar (2008).
- Richard Gaughan, "Laser Technique Monitors Calcium," PHOTONICS SPECTRA, January, p.65 (2002).
- Richard Gaughan, "Near-infrared laser generates calcium waves," Biophotonics International, March, p.54 (2002).
|