

|
| |||
|
|
| ||
|
|
|
| |
|
Laboratory for Scientific Instrumentation and Engineering
|

|
 |
|
||||||||
| Research topics in LaSIE | » Back to Research page |
|
Nano surgery and stimulation of living cells by ultrashort pulsed lasers | |
|
Introduction Near-infrared femtosecond pulsed lasers which induce the process of multi-photon absorption are currently being applied to a variety of applications, for example, three-dimensional microfabrication, ultra-high-density optical data storage, biological imaging, biological cell surgery, and biological stimulation. We are trying to demonstrate nano scale-cellular modification by using femtosecond near-infrared laser pulses in a living cell. When the near-infrared ultrashort pulsed laser is focused into a living cell (especially, intracellular organelle: Mitochondria, ER), the process of multi-photon absorption occurs at the focal spot. Therefore we can fabricate or stimulate the living cell with 3dimensional resolution. Figure 1 shows a schematic image of nano surgery and stimulation of a living cell. Nano surgery by ultrashort pulsed laser [1] Figure 2a) shows a reflection confocal image of four damage sites where submicron-sized nodules were formed at a depth of 2mm below the surface of Rhodamine stained collagen. The low visibility of the point on the right of the image is due to a slight change in the focal plane by the microscope translation stage. These nodules were made at an average power of 7mW with an exposure time of 0.5s ~corresponding to 41×106 pulses of 0.085nJ. This image shows that, even at low average power levels, the focused ultrashort laser pulses are causing real physical damage in the collagen gel, not merely photobleaching of the dye. From this and from other confocal images it appears that the lateral size of the nodules formed is slightly smaller than 1mm. The limit on the minimum size is due to difficulty in observation of subsurface damage sites. Via multiphoton ionization, it should be possible to reduce the nodule size to around 300nm in diameter. Figure 2b) shows a wide field image of the 3D array, with the focal plane at 15mm. The layer to the right of the scale bar is in focus. Qualitatively, no difference was observed between damage sites just beneath the surface and those formed 30mm deep into the specimen. Confocal sectioning in Figure 2a) does not show any influence from adjacent layers, a distance of 2µm away. Nano stimulation of a living cell by ultrashort pulsed lasers [2-6] Figure3 shows a fluorescent image of the intracellular Ca2+ wave in a living HeLa cell (human cervical carcinoma) induced by femtosecond near-infrared laser irradiation. The wavelength, repetition rate and pulse width were 780nm, 82MHz and 80fs, respectively. The average power and exposure time were 50mW and 1/125s, respectively. After the exposure to femtosecond near-infrared laser irradiation, the intracellular Ca2+ concentration ([Ca2+]i) increases at the laser focus. The increase of [Ca2+] i then propagates in the living HeLa cell. The increase of [Ca2+] i looks like a wave, therefore this phenomenon is called an intracellular Ca2+ wave. We can also observe the intercellular propagation of intracellular Ca2+ waves. Ca2+ waves play a role in controlling the cell or organ function, for example, fertilization of eggs, muscle contraction, growth-cone migration, gene transcription, and hormone secretion. This technique allows the possibility of controlling living cell functions by using laser irradiation.
The photogeneration of a calcium response in living cells by laser stimulation allows the possibility of using the laser to periodically trigger calcium elevations in living cells. In heart muscle cells (cardiomyocytes) the calcium concentration controls the cell contraction. We can therefore apply a remote periodic optical trigger and perturb the calcium dynamics in cardiomyocytes. This effect can be seen to act as a pacemaker for the target cell, and even for surrounding cells. By carefully selecting the laser power, periodicity, and phase, we can trigger, entrain, or damp beat frequencies in the cells. This will allow scientists to remotely control single cells inside heart muscle tissue since the near-infrared wavelengths of the laser we use allow deep penetration. The effect can also be seen to propagate through surrounding cells, and may give a better understanding of the mechanisms by which an abnormal beat frequency can propagate through a group of otherwise synchronized cells. This may help to illuminate the physiology of heart-failure at a cellular level.
» Back to Research page |
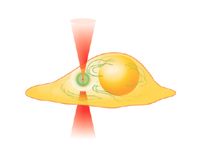 FIg.1 Cell stimulation using ultrashort pulsed laser.
Fig. 4 Laser pacemaking of cardiomyocytes
|