|
Japanese / English
|

|
 ńĀöń®ČÕåģÕ«╣
ńĀöń®ČÕåģÕ«╣ |
 ńĀöń®ČµźŁńĖŠ
ńĀöń®ČµźŁńĖŠ |
 ŃāĪŃā│ŃāÉŃā╝
ŃāĪŃā│ŃāÉŃā╝ |
 Ńé╗Ńā¤ŃāŖŃā╝
Ńé╗Ńā¤ŃāŖŃā╝ |
 LaSIE’╝¤
LaSIE’╝¤ |

|
| Research topics in LaSIE | » Back to Research page |
|
Optical Orientation Processes in Spectrally distinguishable photoisomers | |
|
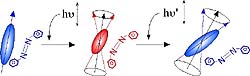
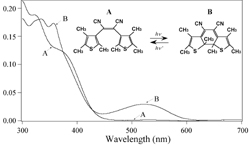
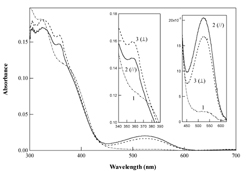
The growing interest that has arisen in photosensitive chromophores containing polymeric films in the past few years has been driven by requirements in the areas of optoelectronics, photonics and optical signal processing. Such materials have been used for holography, optical data storage, integrated optics, molecular switching, all-optical modulation and second- and third-order nonlinear optical effects. Reorientation of photo-excited chromophores alleviates centrosymmetry and isotropy, thereby inducing anisotropy and quadratic and cubic optical nonlinearities (Fig. 1). For fundamental understanding of the reorientation process itself and its correlation with the micro-cogent environment of the chromophore, much of the optically induced reorientation studies have focused on azo dye containing materials. However, despite this activity, studies of light-induced orientation of chromophores other than azobenzene derivatives and azobenzene-type molecules have rarely been reported. In this research we individualize optically oriented photoisomers of diarylethene (DE) and spiropyran (SP) derivatives in thin films of a poly-methyl-methacrylate (PMMA) polymer (Fig. 2). Such photochromic chromophores have been extensively studied, not only from a photochemical point of view, but also for use in near field and three dimensional optical data storage. Besides the technological interest into the photoisomerization of DE and SP derivatives in polymeric thin films, studies of the optical orientation in such systems will improve the comprehension of the reorientation process of photochromic chromophores within isomerization reactions. Indeed, in contrast to azobenzene derivatives, one of the two photochemical isomers of DE and SP type chromophores can be spectrally distinguished, a feature which allows for the individualization of the photo-induced orientation processes in the photo- and thermal isomerization reactions. In this research, we separate the optical orientation processes of both photoisomers of each of the DE and SP chromophores studied, by using the polarized UV-vis spectroscopy technique, and by taking advantage of the natural spectral differences exhibited by the photoisomers in the UV-vis region. Fig. 3 shows the dichroic spectra observed in films of DE/PMMA after linearly polarized UV light irradiation. The insets in Fig. 3 are expanded views of both the UV and visible absorptions of the DE chromophore. These spectra were obtained 30 s after the irradiation (irradiation dose: 78 mJ/cm2). It is clear that the irradiated samples show anisotropic (dichroic) absorbance upon polarized UV irradiation. Identical spectra were recorded for Abs//Āand Abs^Āprior to UV irradiation, demonstrating that the samples were in-plane isotropic at that time. It is particularly remarkable from Fig. 3 that Abs//Āis higher than Abs^Āin the visible band where the absorption of the A form is negligibly small and only the B form exhibits an appreciable absorption. This result was further confirmed by real-time dichroism experiments (see references). This clearly shows that when the DEĀ chromophores undergo the AüsB photoreaction under polarized UV light, the B isomer is formed with its visible transition dipole moment parallel to the UV polarization. Abs^Āis higher than Abs//Āin the UV band; a feature which shows that the UV transition dipole moment of the DE chromophores is perpendicular to the UV polarization after cycles of the AüsB photoisomerization. For both of the DE chromophores, this result shows that the direction of the UV transition of the orientational overlap of the A and B isomers is perpendicular to the visible transition of the B isomer. Photo-selection of DE and SP derivatives is caused by polarized light absorption in proportion to the cosine square of the angle between the transition dipole moment and the polarization of the exciting light. This selective depletion; e.g. photoselection; can create anisotropy by orientational hole burning, OHB, or orientational redistribution, OR, or both. Molecular reorientation can be caused either by photoisomerization processes, or by light-driven rotational diffusion, or both. Some chromophores will not photoisomerize even though they are photo-excited. For DE and SP, the term (re)orientation includes both the reorientation of the chromophore as a block, or block reorientation, and reorientation due to the isomeric shape change without any block rotation, or isomeric reorientation. In principle, isotropy can be restored by rotational diffusion. Both of the A and B photoisomers of DE absorb UV light, and the UV-band anisotropy results from weighted OHB and OR processes which lead to a net orientation of the chromophores UV transition perpendicular to the polarization of the excitation light. References
» Back to Research page |
   |